Keep ahead of the threat
Stay up to date with the latest mycotoxin information by signing up to our newsletter

How Alltech Coppens Successfully Manage Mycotoxins at an Aqua Feed Mill
Authors: Ben Lamberigts, Ruben Groot, Niamh McNally | Alltech Aquaculture
Click below to listen to the Mycotoxin Matters podcast episode with Ben Lamberigts hosted by Martin Minchin. You can also hear the full audio or listen to the episode on Apple Podcasts or Spotify. You can find an edited transcript at the bottom of the page.
The reliance on marine-based ingredients has been problematic for the aquaculture feed industry for many years. The industry still has some ways to go in producing a fish meal-free diet across all species, but R&D developments in this area have highlighted that fish do not require fishmeal to grow and perform optimally. The only requirements are:
- Essential nutrients, such as digestible protein, fat for energy, vitamins and minerals
- Palatable compound feed
- Good water quality
By defining alternatives that meet these requirements, those ingredients are no longer alternatives; they are equivalent — and often superior — sources of nutrition. Plant-based protein sources are still the number-one preferred alternative for replacing fishmeal in aquaculture feeds. The high inclusion of plant-based ingredients in connection with the challenges associated with climate change are expected to bring a higher level of mycotoxin contamination to aquaculture. To act both early and effectively, it is very important to understand the risk level for individual species and specific raw materials, as this is the basis of a strong quality-control program.
A three-pillar approach to quality control
A strong quality control program is the foundation for establishing effective mycotoxin management at the feed mill. A quality control program helps ensure that all raw materials entering the facility are quality-tested based on several factors and strategies in place, including storage, processing and finished feed storage.
At specialist feed mill Alltech Coppens Germany, the establishment of a mycotoxin management program was the result of doctoral research carried out by Vivi Koletsi at the Alltech Coppens Aqua Centre. The initial phase of this project was to assess the risk, and data was collated from the Alltech 37+® laboratory (from 2012 to 2019) comprised of wheat corn and soybean meal and complete fish feeds. A raw material analysis highlighted that more than 80% of the wheat samples, 95% of corn and 87% of soybean returned positive mycotoxin results, 43 individual toxins were detected in wheat and corn and 34 in soybean meal. The analysis of fish feed samples found that DON was the toxin that appeared the most in terms of both occurrence and toxicity.
The second stage of this project led to a meta-analysis completed by Koletsi et al. (2021) to demonstrate the risk of DON on feed intake and growth performance. Simultaneously, data was collected to quantify the risk of exposure in fish. The extent to which DON affects feed intake and growth performance was evaluated by employing a meta-analytical approach.
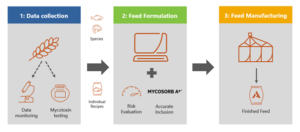
Figure 1: Three-pillar approach to mycotoxin management
Alltech Coppens uses a three-pillar approach to manage the mycotoxin risk. These three pillars are brought together in feed formulation software to analyze and calculate the risk associated with each feed that is being manufactured, with the appropriate practices in place to mitigate the associated risk.
Pillar 1: Mycotoxin data collection
Upon their entry to the feed facility, a mycotoxin risk assessment process to screen the various raw materials of plant origin is in place. This risk assessment is based two factors:
- The individual assessment of raw materials entering the mill. Periodic screening is completed using the Alltech 37+ lab, with more frequent testing performed using the Neogen Raptor for faster results.
- An analysis of the Alltech European Harvest Survey, which is conducted on an annual basis, to assess the risk for raw materials in the region. This risk assessment process reveals which raw materials are at risk for containing mycotoxins and which mycotoxins are present in these raw materials.
Pillar 2: Feed formulation
In addition to these assessments, there are FDA and EU legal limits for aflatoxins and guidelines on the safe levels of mycotoxins in aquafeed. As such, we take these different tolerance levels for specific mycotoxins in different species into account when formulating the feed for different fish species.
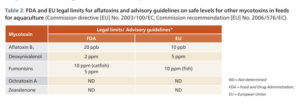
There are over 50 different types of mycotoxins, but the most common and prevalent is deoxynivalenol (DON). As stated previously, the meta-analysis completed by Koletsi et al. (2021) highlights the risk of DON to feed intake and performance, and data was also collected to quantify the risk of exposure in fish. This approach highlighted that the current recommendations for the limit of DON in fish diets are too high and need to be reviewed to protect fish welfare and business profitability.
Pillar 3: Feed manufacturing
This information provides an estimated mycotoxin risk of the raw materials and the sensitivity of the targeted species (although it’s the combination of the raw materials and their specific inclusion level that determines the total risk of the feed). Using feed formulation software, the risk equivalent (REQ) is calculated. This is done by comparing the calculated mycotoxin levels of the recipe against the sensitivity of the fish species. With this REQ, the mycotoxin binder inclusion is calculated based on a linear correlation. The main advantage of this system is that the risk is both recipe- and species-specific and is calculated very accurately, meaning the mycotoxin binder is used at the most efficient ratio.
Importance of continuous testing
To accurately assess the risk in aquaculture, continuous testing is crucial. Recently, two separate surveys have been conducted — one in Norway and another regional survey in Asia-Pacific.
In the Norway study, a total of 104 samples were collected over a period of 12 months from two salmon farms: Ellingsen Seafood (high fish meal inclusion) and Blom Fiskeoppdrett (low fishmeal inclusion). The samples were sent to an independent laboratory in Belgium for mycotoxin analysis. The results showed that no mycotoxin contamination was present as a result of good quality control programs in place at the associated feed mills.
Meanwhile, in Asia-Pacific, we conducted a survey to determine the mycotoxin risk in the region. Almost 200 samples were collected from Bangladesh, China, India, Indonesia, Malaysia and Vietnam and were tested utilizing our Alltech 37+® analytical laboratories or locally using the Alltech RAPIREAD® system by utilizing Neogen’s lateral-flow technology. The samples combined complete feeds and the most commonly used aquafeed raw materials. Most of the raw materials, such as corn, wheat and soybean, were imported from either Europe or the Americas.
The results of the study revealed that byproducts of corn and wheat were quite high in mycotoxin contamination, but more importantly, they highlighted significant differences between the highest results and the average results, emphasizing the importance of continuous testing. Additionally, the combined synergistic effects of multiple mycotoxins over a long ingestion period can be more harmful. This can impact feed intake, growth rate and mortality rates and lead to severe economic losses for farmers.








